Apoptosis Signaling Pathway
Click on the links shown in the Explore Pathways box below to highlight the factors involved in either the extrinsic or intrinsic pathway of caspase activation.
Death Receptor Activation
Death Receptor Activation
Pro-Caspase-10
Pro-Caspase-10
Pro-Caspase-10
Pro-Caspase-10
Pro-Caspase-10
Pro-Caspase-10
TRAIL R2
TRAIL R2
Pro-Caspase-10
Pro-Caspase-10
Pro-Caspase-10
Pro-Caspase-10
Caspase-10
Caspase-10
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
HTRA2/Omi
HTRA2/Omi
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
DNA Damage
DNA Damage
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Cleavage of Target Molecules
Cleavage of Target Molecules
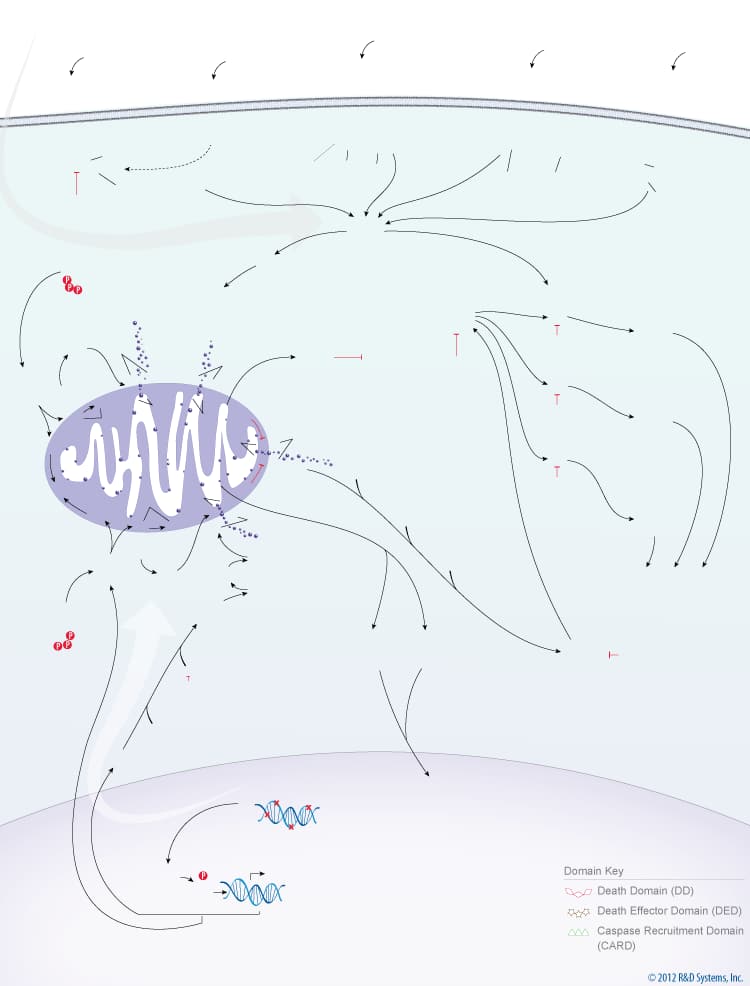
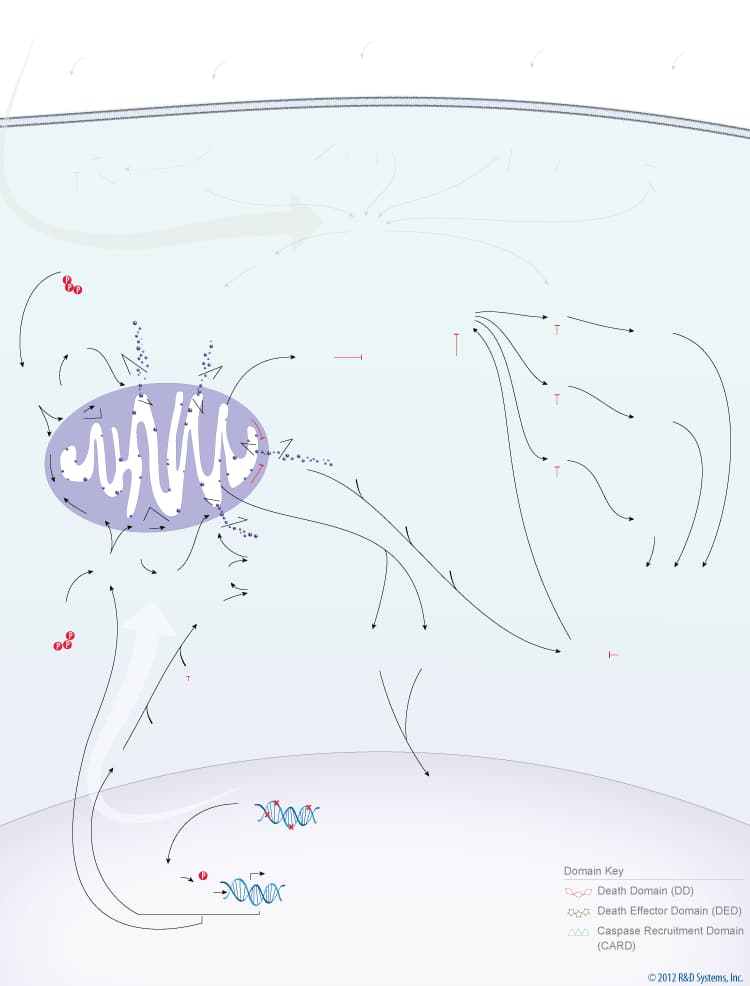
Overview of the Extrinsic and Intrinsic Pathways of Caspase Activation
Caspases are a family of aspartate-specific, cysteine proteases that serve as the primary mediators of apoptosis. All caspases are synthesized as inactive zymogens containing a variable length pro-domain, followed by a large (20 kDa) and a small subunit (10 kDa). Caspase activation occurs following receipt of an extrinsic or intrinsic death signal. The extrinsic pathway of caspase activation is initiated by ligand binding to cell surface death receptors, such as TNF RI, Fas/CD95, DR3, TRAIL R1/DR4, or TRAIL R2/DR5.Ligand binding to these receptors leads to receptor oligomerization and recruitment of FADD and/or TRADD, two death domain-containing adaptor proteins. FADD subsequently recruits Pro-Caspase-8 and Pro-Caspase-10 through its death effector domain. Clustering of pro-caspases in the cytosol near a death receptor leads to formation of the death-inducing signaling complex (DISC) and the subsequent cleavage of Pro-Caspase-8 and Pro-Caspase-10.
The intrinsic pathway of caspase activation is initiated by events such as DNA damage, growth factor withdrawal, or loss of contact with the extracellular matrix. These events ultimately lead to changes in the integrity of the mitochondrial membrane, which is regulated by Bcl-2 family proteins. The balance between pro- and anti-apoptotic Bcl-2 family members determines whether or not a cell will undergo apoptosis. In healthy cells, phosphorylated Bad is sequestered in the cytoplasm by the 14-3-3 protein, and Bcl-2 and Bcl-xL bind to the pro-apoptotic Bax and BAK proteins to inhibit apoptosis. When cytoplasmic levels of free Bad increase, Bcl-2 and Bcl-xL bind to Bad and release Bax and BAK. Bax and BAK, or processed forms of these proteins, can then insert into the mitochondrial membrane, compromising its integrity. Loss of mitochondrial integrity results in the release of pro-apoptotic proteins including Cytochrome c, Smac/Diablo, HTRA2/Omi, Apoptosis-Inducing Factor (AIF), and Endonuclease G. In the cytoplasm, Cytochrome c interacts with APAF-1, which recruits Pro-Caspase-9 to form the apoptosome. Within this complex, Caspase-9 is processed and activated. The intrinsic pathway of caspase activation can also lead to Caspase-2 activation. Following DNA damage, p53 induces the expression of PIDD (p53-induced protein with a death domain), which associates with the CRADD/RAIDD adaptor protein and Pro-Caspase-2 to form the PIDDosome. Formation of this complex leads to the cleavage and activation of Pro-Caspase-2. Once activated, the initiator caspases, Caspase-2, Caspase-8, Caspase-9, Caspase-10, cleave downstream effector caspases, Caspase-3, Caspase-6, Caspase-7, which then promote the ordered disassembly of the cell by targeting a number of critical cellular proteins, including structural proteins, DNA repair proteins, and proteins involved in signal transduction pathways.
To learn more, visit our Apoptosis Research Area.
Get Print Copy of this Pathway